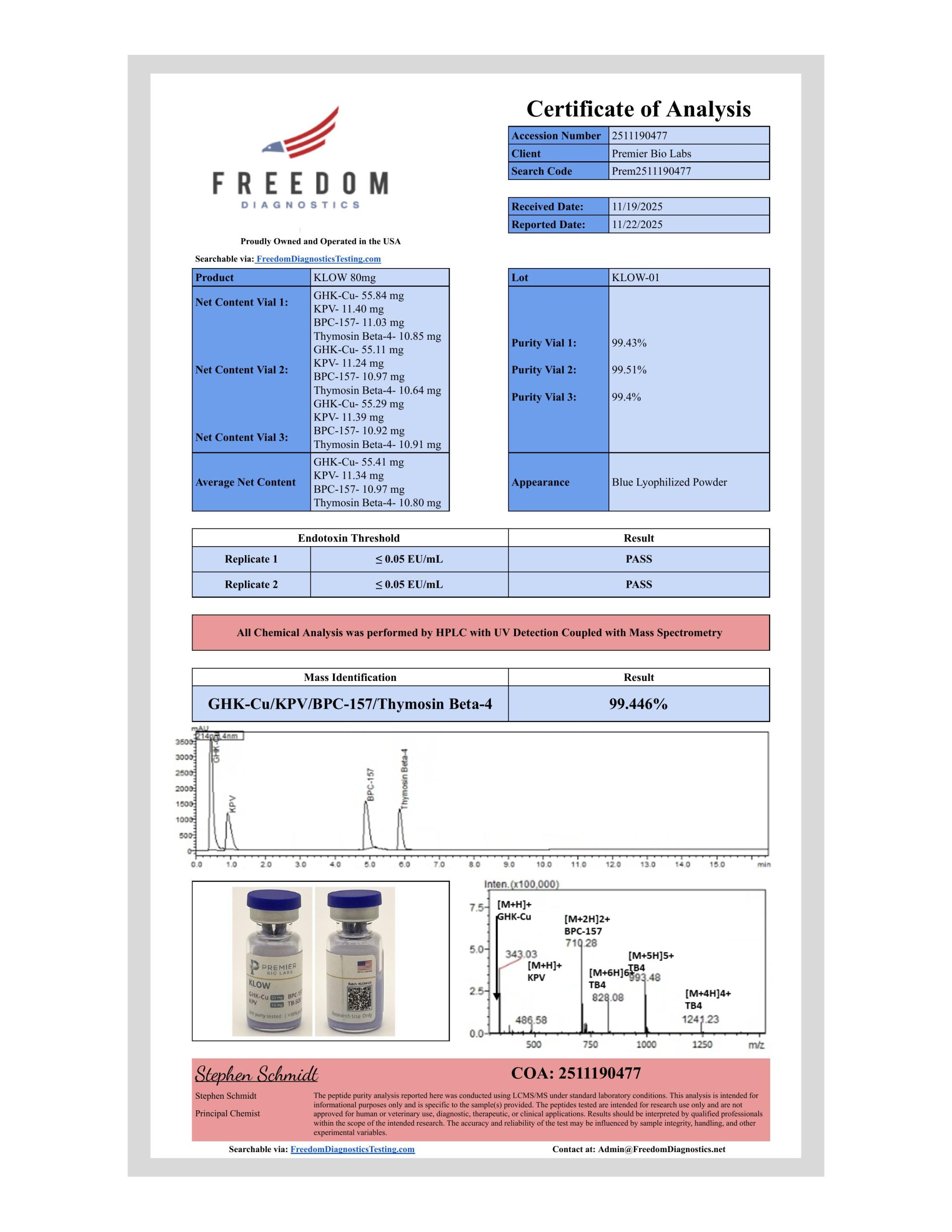
What Is a Certificate of Analysis?
A Certificate of Analysis (CoA) is a documented report from a laboratory that verifies a product meets its stated specifications. For research peptides, a CoA confirms the compound’s identity, purity, and quality through standardized analytical testing.
Think of a CoA as a peptide’s fingerprint and health report combined. It tells you exactly what’s in the vial, how pure it is, and whether it matches what should be there.
For researchers, the CoA isn’t optional documentation—it’s essential verification that directly impacts experimental validity and reproducibility. Using peptides without proper CoA documentation introduces unknown variables that can compromise months of research.
Why Certificates of Analysis Matter
Research Reproducibility
Science depends on reproducibility. When a study uses “BPC-157” without verified purity and identity, the results become difficult to replicate. Was the observed effect from the peptide—or from the 15% impurities present in an untested product?
Published research typically specifies peptide purity and supplier for exactly this reason. Reviewers and other researchers need assurance that materials meet minimum quality standards.
Experimental Validity
Impurities aren’t inert. They can:
- Produce confounding biological effects
- Interfere with assay measurements
- Cause unexpected cytotoxicity
- Alter solubility and stability profiles
A 95% pure peptide contains 5% “something else”—and that something else matters.
Safety Considerations
Research materials interact with biological systems, equipment, and personnel. Unknown impurities—residual solvents, heavy metals, endotoxins, or synthesis byproducts—introduce unquantified risks.
Investment Protection
Quality peptides represent significant investment. Researchers allocating budget to materials deserve verification that they’re receiving what they paid for—not degraded, mislabeled, or substandard product.
Anatomy of a Certificate of Analysis
A complete CoA contains several key sections. Understanding each component helps researchers evaluate quality and identify potential issues.
Header Information
Product Identification
- Product name (e.g., “BPC-157 Acetate”)
- Catalog or product number
- CAS number (unique chemical identifier)
Batch Information
- Lot number or batch number
- Manufacturing date
- Expiration date (if applicable)
Supplier Information
- Company name
- Contact information
- Quality assurance signatures
This header information enables traceability. If questions arise about a specific product, the lot number allows investigation of that exact batch.
Key Tests on a Peptide CoA
HPLC Purity Testing
What It Is: High-Performance Liquid Chromatography (HPLC) is the gold standard for peptide purity assessment. The technique separates compounds based on their chemical properties, allowing quantification of the target peptide versus impurities.
What You’ll See:
Test: HPLC Purity
Method: RP-HPLC, C18 column, UV detection at 220nm
Specification: ≥98.0%
Result: 99.2%
Status: PASS
How to Interpret:
| Purity Level | Interpretation |
|---|---|
| >99% | Excellent—premium research grade |
| 98-99% | Standard research grade |
| 95-97% | Acceptable for some applications |
| <95% | Generally unsuitable for research |
What the Chromatogram Shows:
Many CoAs include the actual HPLC chromatogram—a graph showing peaks at different retention times. The main peak represents the target peptide; smaller peaks represent impurities.
A quality chromatogram shows:
- One dominant, sharp peak (target peptide)
- Minimal smaller peaks (low impurity levels)
- Clean baseline (no broad humps or noise)
- Clear peak integration values
Red Flags:
- No chromatogram provided (just a number)
- Multiple large peaks of similar size
- Broad, poorly resolved peaks
- Purity stated without methodology
Mass Spectrometry (MS)
What It Is: Mass spectrometry confirms the identity of a compound by measuring its molecular weight. For peptides, this verifies that the synthesis produced the correct sequence—not a truncated, modified, or entirely different peptide.
What You’ll See:
Test: Mass Spectrometry (ESI-MS or MALDI-TOF)
Specification: 1419.5 ± 1.0 Da
Observed Mass: 1419.6 Da
Status: PASS
How to Interpret:
The observed mass should match the theoretical molecular weight for the peptide. Small variations (±1-2 Da) are acceptable due to instrument calibration and isotope patterns.
| Scenario | Interpretation |
|---|---|
| Observed matches expected | Correct peptide identity confirmed |
| Observed is lower | Possible truncation or deletion |
| Observed is higher | Possible adduct, modification, or wrong peptide |
| Multiple masses | Mixture or degradation present |
Why This Matters:
HPLC tells you purity; MS tells you identity. A vial could be 99% pure—but 99% pure wrong peptide. Mass spectrometry catches synthesis errors, labeling mistakes, and substitutions.
Red Flags:
- No MS data provided
- Mass significantly different from expected
- Multiple masses without explanation
- Generic “confirmed” statement without actual values
Amino Acid Analysis (AAA)
What It Is: Amino acid analysis breaks down the peptide into its constituent amino acids and quantifies each one. This confirms the peptide contains the correct amino acids in the correct ratios.
What You’ll See:
Test: Amino Acid Analysis
Expected: Gly(3), Glu(1), Pro(4), Lys(1), Ala(2), Asp(2), Leu(1), Val(1)
Observed: Within ±10% of expected ratios
Status: PASS
How to Interpret:
Results typically show each amino acid with expected and observed ratios. Values should fall within acceptable tolerance (usually ±10%) of theoretical composition.
When It’s Critical:
- Novel or custom peptides
- High-stakes research applications
- Verification of specific sequences
Not all CoAs include AAA—it’s more common for custom synthesis or premium products.
Peptide Content
What It Is: Peptide content indicates what percentage of the vial’s total weight is actual peptide versus salts, water, and counterions. A “5mg” vial doesn’t always contain 5mg of peptide.
What You’ll See:
Test: Peptide Content (by AAA or nitrogen analysis)
Result: 82.5%
Net Peptide: 4.1 mg (in 5mg gross weight vial)
How to Interpret:
Lyophilized peptides typically contain:
- 70-85% peptide content (common)
- 85-95% peptide content (high)
- Remainder: counterions (acetate, TFA), residual moisture, salts
This matters for accurate dosing in research protocols. A vial labeled “5mg” with 80% peptide content actually contains 4mg of peptide.
Red Flags:
- Peptide content not stated
- Unusually high claims (>95% is rare)
- Gross weight presented as net peptide weight
Appearance and Solubility
What It Is: Basic physical characterization confirming the peptide looks and behaves as expected.
What You’ll See:
Test: Appearance
Specification: White to off-white lyophilized powder
Result: White lyophilized powder
Status: PASS
Test: Solubility
Specification: Soluble in water at 1 mg/mL
Result: Clear, colorless solution
Status: PASS
How to Interpret:
Most research peptides should be:
- White to off-white powder (yellow may indicate degradation)
- Freely soluble in water or specified solvent
- Clear solution without particulates after reconstitution
Additional Quality Tests
Depending on the supplier and application, CoAs may include:
Residual Solvents
- Tests for leftover synthesis solvents (acetonitrile, TFA, DMF)
- Important for sensitive applications
- Results typically in ppm (parts per million)
Water Content (Karl Fischer)
- Measures residual moisture in lyophilized powder
- Affects stability and peptide content calculations
- Typically <10% for properly lyophilized peptides
Endotoxin Testing (LAL)
- Detects bacterial endotoxins
- Critical for in vivo research applications
- Results in EU/mg (endotoxin units per milligram)
Bioburden / Sterility
- Confirms absence of microbial contamination
- Important for cell culture and in vivo work
Heavy Metals
- Screens for lead, mercury, arsenic, cadmium
- Ensures synthesis and handling quality
How to Read a CoA: Step-by-Step
Step 1: Verify Product Identity
Confirm the CoA matches your product:
- Product name matches your order
- CAS number is correct for the peptide
- Lot number matches your vial label
Mismatch = wrong CoA. Request the correct documentation.
Step 2: Check the Date
- When was testing performed?
- Is there an expiration date?
- Is the CoA recent relative to your purchase?
Old CoAs for new batches suggest the supplier is recycling documentation rather than testing each lot.
Step 3: Evaluate Purity (HPLC)
- Is purity ≥98% for research applications?
- Is the testing method specified?
- Is a chromatogram included?
Step 4: Confirm Identity (MS)
- Does observed mass match expected molecular weight?
- Is the mass within acceptable tolerance (±1-2 Da)?
- Are actual values provided (not just “confirmed”)?
Step 5: Review Additional Tests
- Are tests appropriate for your application?
- Do all results show “PASS”?
- Are specifications reasonable and clearly stated?
Step 6: Assess Overall Quality
- Is the document professional and complete?
- Is testing performed by the supplier or third party?
- Can you contact the laboratory if questions arise?
Third-Party vs. In-House Testing
In-House Testing
The supplier performs their own quality control testing.
Advantages:
- Faster turnaround
- Lower cost (passed to customer as lower prices)
- Immediate quality feedback during production
Disadvantages:
- Potential conflict of interest
- Equipment calibration questions
- No independent verification
Third-Party Testing
An independent laboratory performs testing.
Advantages:
- Unbiased, independent verification
- Specialized expertise and equipment
- Greater accountability and traceability
- Industry credibility
Disadvantages:
- Additional cost
- Longer turnaround time
- Requires supplier commitment to quality
Best Practice:
Premium research suppliers use third-party testing for final release or provide third-party verification alongside in-house QC. This combination offers both speed and independence.
Red Flags: When to Question a CoA
Document Quality Issues
| Red Flag | What It Suggests |
|---|---|
| Generic template with no batch specifics | Recycled or fabricated documentation |
| Missing lot/batch number | Cannot trace to your specific product |
| No testing dates | Age and validity uncertain |
| Blurry or low-resolution images | Possibly altered or copied |
| Missing company information | Accountability concerns |
Testing Deficiencies
| Red Flag | What It Suggests |
|---|---|
| HPLC purity without chromatogram | Number may be fabricated |
| “Confirmed” without actual MS value | Identity not properly verified |
| No methodology stated | Results not reproducible or verifiable |
| Specifications missing | No quality standard defined |
| Only one test performed | Incomplete quality assessment |
Suspicious Results
| Red Flag | What It Suggests |
|---|---|
| Purity exactly 99.0% every batch | Template recycling, not actual testing |
| Results always just above specification | Possible data adjustment |
| Mass significantly off expected value | Wrong peptide or degradation |
| Chromatogram shows multiple large peaks | Significant impurities present |
Supplier Behavior
| Red Flag | What It Suggests |
|---|---|
| Cannot provide CoA before purchase | Quality not prioritized |
| Different CoA for same lot number | Fraudulent documentation |
| Refuses questions about testing | Lack of transparency |
| Cannot provide third-party verification | Testing claims unverifiable |
Questions to Ask Your Supplier
When evaluating a peptide supplier, request clear answers to:
- “Can I see a sample CoA before ordering?”
- Reputable suppliers share documentation openly
- “Is testing performed in-house or by a third party?”
- Know who’s making quality claims
- “Will I receive a batch-specific CoA with my order?”
- Generic CoAs don’t verify your specific product
- “What purity specification do you guarantee?”
- “High purity” is meaningless; ≥98% is a standard
- “Can I contact the testing laboratory with questions?”
- Legitimate testing should be traceable
- “What happens if my product doesn’t match the CoA?”
- Quality suppliers stand behind their documentation
Understanding Common Peptide Impurities
When reviewing CoAs, understanding what impurities mean helps assess significance:
Synthesis-Related Impurities
Truncated Sequences (Des-peptides)
- Missing one or more amino acids
- Result from incomplete synthesis steps
- May have partial biological activity
Deletion Sequences
- Internal amino acid missing
- Harder to detect than truncations
- Can significantly alter function
Racemization Products
- Amino acid chirality flipped (D- instead of L-)
- Can affect biological recognition
- Cumulative with each synthesis step
Process-Related Impurities
Residual TFA (Trifluoroacetic acid)
- Common counterion from HPLC purification
- Can affect pH and cell viability at high levels
- Acetate exchange reduces TFA content
Residual Solvents
- Acetonitrile, DMF, DCM from synthesis
- Should be below ICH guidelines
- Important for in vivo applications
Scavenger Residues
- From cleavage and deprotection steps
- Various organic compounds
- Usually removed by purification
Degradation Products
Oxidation Products
- Methionine → methionine sulfoxide
- Tryptophan → various oxidized forms
- Indicate improper handling or storage
Deamidation Products
- Asparagine → aspartic acid
- Glutamine → glutamic acid
- Increase with time, temperature, pH
Aggregation
- Peptide molecules clumping together
- May appear as multiple peaks or broad peaks
- Indicates stability issues
Frequently Asked Questions
What purity should I look for in research peptides?
For standard research applications, seek ≥98% HPLC purity. Premium applications may warrant ≥99%. Below 95% is generally unsuitable for research due to significant impurity levels.
Why do some CoAs not include a chromatogram?
Some suppliers omit chromatograms to simplify documentation or obscure quality issues. Chromatograms provide transparency—their absence should prompt questions about testing validity.
What’s the difference between purity and peptide content?
Purity (HPLC) measures the target peptide versus other peptide-related impurities. Peptide content measures actual peptide versus total weight (including salts, water, counterions). Both matter for accurate research.
How do I verify a CoA is legitimate?
Check for batch-specific information, complete methodology, actual test values (not just “pass/confirmed”), professional formatting, and contact information. When in doubt, contact the supplier’s quality department with specific questions.
Should I request a CoA before purchasing?
Yes. Reputable suppliers provide sample CoAs upon request. Reviewing documentation before purchase ensures quality expectations align and identifies potential issues early.
What does “research grade” actually mean?
No universal standard exists for “research grade.” Look for specific purity specifications (≥98% HPLC) rather than vague quality claims. Terms like “pharmaceutical grade” require verified GMP manufacturing—rare for research peptides.
How often should suppliers test their peptides?
Each manufactured batch should receive independent testing. CoAs should be batch-specific with unique lot numbers. Suppliers providing identical CoAs across different batches aren’t testing each lot.
The Premier Bio Labs Standard
Transparency and quality aren’t marketing claims, they’re measurable commitments. Every peptide from Premier Bio Labs includes:
Batch-Specific Documentation
- Unique lot numbers for full traceability
- Testing performed on your specific batch
- Complete documentation with every order
Third-Party Verification
- Independent laboratory testing
- Unbiased quality confirmation
- Verifiable results
Comprehensive Testing
- HPLC purity analysis with chromatograms
- Mass spectrometry identity confirmation
- Additional testing as appropriate
Guaranteed Specifications
- Minimum ≥98% purity standard
- Clear specifications for every product
- Quality guarantee backed by documentation
Full Transparency
- Sample CoAs available before purchase
- Questions welcomed and answered
- Quality team accessible for technical inquiries
Your research deserves materials you can trust. We provide the documentation to prove it.